Project Name
Continued Development of High Melt Fluidity Clear
Project Codenumber
UnAssigned
Notes
This overview was updated July 21, 2022, it has new insights.
I have used Cone 6 G3806C (as a copper blue) for some time but in 2019 it was time to take the thermal expansion down further to make it work on all the bodies I use. It turned out to be a lot further than I thought! The final recipe ends up being much more expensive, but I judge this as worthwhile for a glaze with so much potential.
In this series I did adjustments (some of which are shown here), step-by-step, inching each toward lower calculated thermal expansion while trying to hold on to the high melt fluidity and brilliant surface. I targeted being able to survive a 325F-to-icewater test without crazing on my hardest-to-fit body, Plainsman P300. It took many more trials than I thought it would.
When crazing occurs the spotlight is almost always on K2O+Na2O (or KNaO). These are great fluxes to melt glaze and get super gloss but they have super-high thermal expansions. By substituting as much of the KNaO for other fluxes as possible I wanted to effect a reduction in the calculated thermal expansion while holding on to the fired appearance and properties (materials in the recipe source the oxides, so judicious recipe adjustments can be done to effect chemistry changes).
Achieving durability yet with a fluid melt was the key objective. So I wanted to maintain the Al2O3 level around 0.3. I wanted to replace the KNaO with multiple fluxing oxides (e.g. Li2O, ZnO, MgO, SrO) to get the "mixed oxide effect", known to produce better melting. I wanted to maintain the B2O3 as low as possible to avoid excessive fluidity and bubbling issues.
On each test cycle I proposed a change through chemistry calculations, tested it thoroughly, then proposed a new one based on the observations. As it turned out, sourcing the B2O3, KNaO and portions of other oxides from frits was key to getting a crystal clear glass.
This project ended up taking two tracks. The first ends at G3806E. That keeps the B2O3 at 0.11 and achieves the fluid melt using the mixed oxide effect (thus the wide range of materials in the recipe). It has a minimum of frit and the frits are common. This is the recommended recipe for most. That being said, the blue color has been lost, it is now green!
Track number two increases the amount of frit being used and employ 3-5 times more B2O3. The extra melt from that enabled greatly increasing the Al2O3 and SiO2 levels (for durability), but almost doubling it! And increasing the SiO2 much more also. In the end I got a glaze I thought was impossible to make: A super glossy high melt fluid that is durable and of very low thermal expansion. By simply increasing the kaolin it can be moved toward a more general purpose base (like G2926B I normally use but with a lower thermal expansion). By reducing the kaolin it can be made even more fluid. All while maintaining the same COE.
Any of these variations could be employed since the ultimate lowest thermal expansion version may not be needed for your body. Starting with the "K" version we are using frits that you likely cannot get. That means that, for now, the G3806E is the most practical.
Panama Cone 6 Adjustment 2015
High fluid melt glaze for reactive effects and super gloss colors
|
Code # G3806C |
| P | Materials | Amt | |
|---|---|---|---|
| Silica | 26.300 | 26.27% | |
| Kaolin | 19.700 | 19.68% | |
| Dolomite | 8.700 | 8.69% | |
| Strontium Carbonate | 4.400 | 4.40% | |
| Ferro Frit 3110 | 31.100 | 31.07% | |
| Ferro Frit 3134 | 6.600 | 6.59% | |
| Zinc Oxide | 3.300 | 3.30% |
| P | Additions | ||
|---|---|---|---|
| * | Copper Oxide | 2.000 | 2.00% |
| * | Tin Oxide | 2.500 | 2.50% |
Total:104.60
Auto Unity Formula
|
Si:Al: 11.1:1 7.3 (Molar:7.1) 7.9 |
Notes
This is work I did in 2015 (in 2019 a much bigger project developed this further).
The copper and tin produce the turquoise celadon effect.
This recipe is for a brilliant fluid-melt transparent base glaze, initially for copper blues and greens, but later for stains. "Fluid-melt" means it runs down off ware if applied too thickly, this is a key for achieving many visual effects.
Initiailly I compared a number of recipes I found on line and finally selected Panama Blue. I removed the colorants and made adjustments to improve slurry properties and lower the thermal expansion (it has serious crazing issues). Fluid-melts have a down side: Crazing is an issue (because the fluid melt requires more fluxes, these have higher thermal expansions).
Then I did three adjustments, each lowering the thermal expansion more than the last. While keeping the same brilliant visual appearance. The recipe ended up being quite different (two materials were eliminated from the recipe, their oxides supplied by the others). The chemistry of this one moves much of the KNaO to low-expansion MgO. This makes it melt a little less, but visually it is the same. Higher ZnO helps melting (since MgO is not nearly as powerful a flux as KNaO). I was even able to add extra SiO2. The calculated thermal expansion has gone from 7.7 down to 7.3.
This worked well on stonewares but still crazed on Plainsman P300 and M370 (but was OK on Polar Ice). Fluid melt glazes look best on porcelains so this was obviously a problem. So I continued development in pursuit of a fluid melt having a lower thermal expansion (see subsequent articles, recipes and posts).
Pictures
Copper Blue G8306C using copper carbonate, oxide

Right is G3806C, an adjustment to drop the thermal expansion of B. It does this by trading some of the high-expansion KNaO for a mix of MgO, ZnO and SrO. These is an improvement but it still could craze over time on high-kaolin or low silica porcelains.
One more change: The one on the right uses 2% Copper Oxide instead of 2% Copper Carbonate (left). Both also add 2.5% tin oxide. Strangely the color is only slight darker (the oxide is a more concentrated form of copper than the carbonate).
Plainsman P300, M370 with copper blue glaze cone 6

This is the G3906C base plus 2.5% tin oxide and 2% copper oxide. The green glaze does craze over time on these bodies, but the inside glaze is a liner than will not.
3806C vs. other cone 6 clear glazes on a dark stoneware
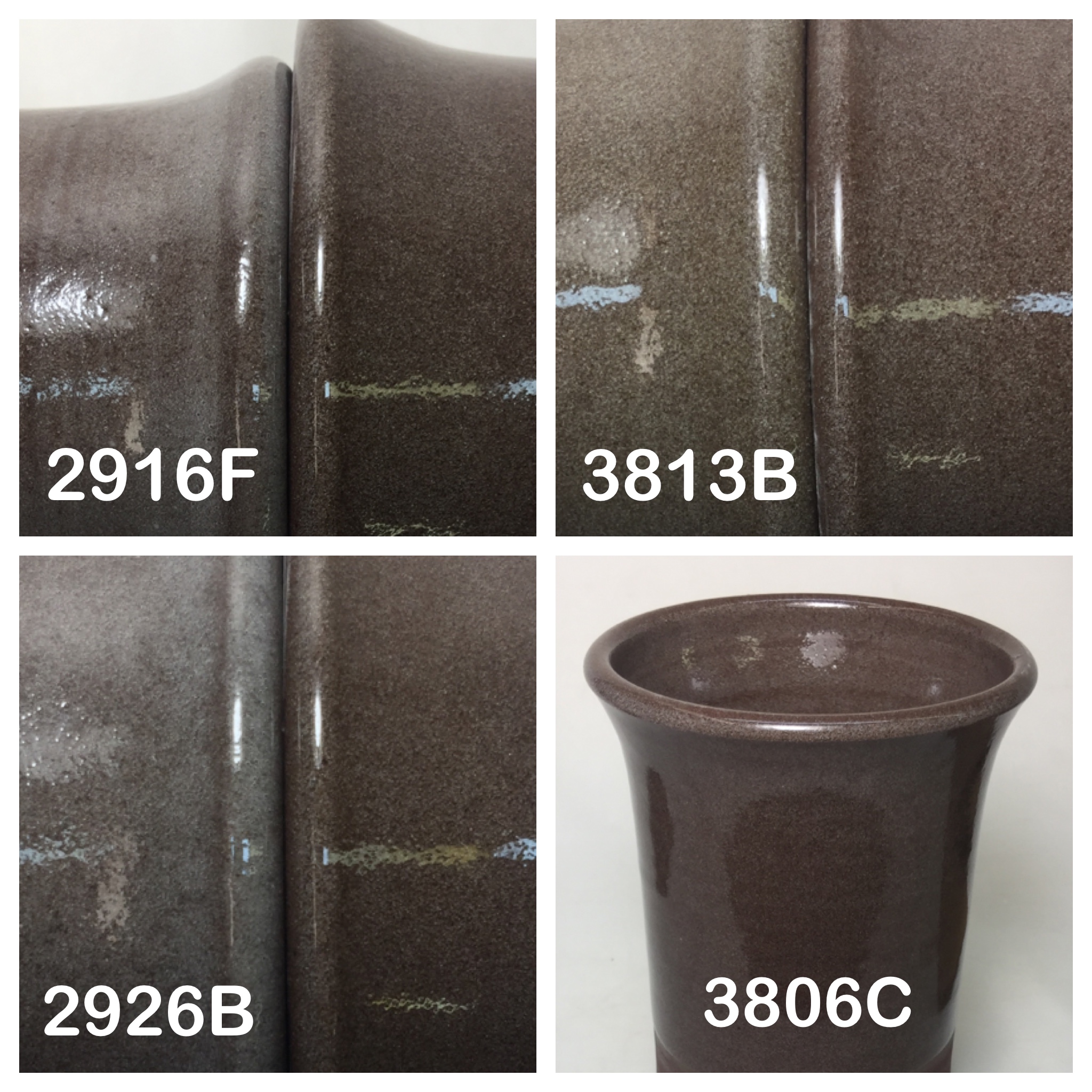
Each pair of mugs shows a numbered glaze vs. G3806C on the right. The body is a red burning cone 6 stoneware, Plainsman M390.
G2926B, 3806C vs. Amaco C11 Clear at cone 6

Bottom right is P300 with three coats of C11.
Bottom left: 10 gram ball of C11.
2926 B is top left, 3806C is top right.
G3806C Copper Blue on Polar Ice

Polar Ice is the easiest of Plainsman middle fire porcelains to fit a glaze to, although this glaze crazes on most other porcelains, it should stay craze free on this.
G3806C on a dark burning cone 6 stoneware

Plainsman M390. There is still some clouding, but it is better than other transparents we have used.
G3806D melt flow test

Left is G3806C with copper oxide 2%. Right is G3806D with copper carbonate 2%. The melt fluidity is identical. The blue color thus seems to depend on the carbonate (or a lower percentage of the oxide is needed).
Variations
1 - Midnight
Fire fast to 2100F (300-400F/hr), then 100F/hr to 2200F, then drop fast to 2000F and soak half hour, then cool at 100F/hr to 1400F.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Cone 6 Adjustment 2015" keywords="High fluid melt glaze for reactive effects and super gloss colors" id="75786" key="JkiQ1k4w" date="2024-07-24" codenum="G3806C" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="26.300" tolerance=""/> <recipeline material="Kaolin" amount="19.700" tolerance=""/> <recipeline material="Dolomite" amount="8.700" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.400" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="31.100" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="6.600" tolerance=""/> <recipeline material="Zinc Oxide" amount="3.300" tolerance=""/> <recipeline material="Copper Oxide" amount="2.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2024-07-24 17:59:11
Panama c6 - Lower COE #1
|
Code # G3806D |
| P | Materials | Amt | |
|---|---|---|---|
| Silica | 26.300 | 26.27% | |
| Wollastonite | 4.000 | 4.00% | |
| Kaolin | 15.700 | 15.68% | |
| Dolomite | 5.700 | 5.69% | |
| Strontium Carbonate | 4.400 | 4.40% | |
| Ferro Frit 3110 | 29.100 | 29.07% | |
| Ferro Frit 3249 | 5.600 | 5.59% | |
| Zinc Oxide | 3.300 | 3.30% | |
| Spodumene | 6.000 | 5.99% |
| P | Additions | ||
|---|---|---|---|
| * | Copper Carbonate | 2.000 | 2.00% |
| * | Tin Oxide | 2.500 | 2.50% |
Total:104.60
Auto Unity Formula
|
Si:Al: 10.8:1 6.9 (Molar:6.6) 6.0 |
Notes
The first adjustment to G3806C, the recipe I have used by some years. I have reduced KNaO and raised Li2O to compensate (Li2O is a powerful flux and has very low expansion). The method to accomplish this is novel.
-Some of the KNaO and almost all of the B2O3 were coming from frit 3134. I removed that and introduced frit 3249. It has no KNaO, plenty of B2O3. But it also has MgO (another low expansion flux). The portion of the MgO that I can supply in-the-frit will melt much better than it does when supplied from dolomite.
-I am sourcing the Li2O from spodumene. Not much is needed because I only need 0.05 molar.
The chemistries of the copper and tin are turned off so they are not participating.
The results have been stunning so far. The fired appearance is almost identical. However it is still crazing on Plainsman P300.
Mixed 2700 water to 3000 dry to get 1.45 SG. Does not respond well to Epsom salts, even at 2g/1000. Slurry is pretty runny but does work fine on quick dips with 1850F bisque. Needs some bentonite to increase particle surface area.
Pictures
G2806D (with copper carbonate) on M340 at cone 6

G3806D melt flow test

Left is G3806C with copper oxide 2%. Right is G3806D with copper carbonate 2%. The melt fluidity is identical. The blue color thus seems to depend on the carbonate (or a lower percentage of the oxide is needed).
G3806D with 2% Copper Carb (left), 2% Copper Ox (right)

G3806C and D - both using Copper Oxide at 2%

G3806D on M340, Polar Ice at cone 6

G3806D on M340, M390 at cone 6

This is the copper oxide version.
Clockwise: G3806C,D,E,F on M370

F is more fluid, thus highlights contours better.
C has 2% copper, the others 3%.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama c6 - Lower COE #1" id="157007" key="eaXjs1mN" date="2024-01-18" codenum="G3806D" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="26.300" tolerance=""/> <recipeline material="Wollastonite" amount="4.000" tolerance=""/> <recipeline material="Kaolin" amount="15.700" tolerance=""/> <recipeline material="Dolomite" amount="5.700" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.400" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="29.100" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="5.600" tolerance=""/> <recipeline material="Zinc Oxide" amount="3.300" tolerance=""/> <recipeline material="Spodumene" amount="6.000" tolerance=""/> <recipeline material="Copper Carbonate" amount="2.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2024-01-18 19:45:42
Panama c6 - Lower COE #2
|
Code # G3806E |
| P | Materials | Amt |
|---|---|---|
| Silica | 32.000 | |
| Spodumene | 11.000 | |
| Zinc Oxide | 3.000 | |
| Ferro Frit 3249 | 6.000 | |
| Ferro Frit 3110 | 22.000 | |
| Strontium Carbonate | 5.000 | |
| Kaolin | 11.000 | |
| Bentonite | 2.000 | |
| Dolomite | 8.000 |
| P | Additions | |
|---|---|---|
| * | Copper Oxide | 4.000 |
| * | Tin Oxide | 2.500 |
Total:106.50
Auto Unity Formula
|
Si:Al: 11.4:1 6.3 (Molar:6.0) 6.9 |
Notes
This takes the calculated COE even lower (6.9 of G3806D to 6.3). I have increased Li2O, MgO at the expense of KNaO and CaO.
This produces a stunning copper blue/green. While it is still failing the 300F/ice-water test on P300, the calculated thermal expansion of 6.3 (vs. the original 7.3 in G3806C) means that this would fit many bodies. However if 45 micron silica was used more would go into solution in the melt and glaze fit would improve.
The advantage that this has over subsequent and previous versions is:
-It has kept the B2O3 low (a glassy smooth flow with no bubbling)
-It does not need an exotic frit, it works with just spodumene to source the Li2O and strontium carbonate for the SrO. While some of the MgO is sourced from a frit, most is still coming from inexpensive dolomite.
-The total frit percentage is only 28%.
Pictures
G3806E on Polar Ice - Cone 6

I think you will agree, this is stunning! And the melt fluidity is about right: I have it on pretty thick and yet it has not run on to the kiln shelf. Can you imagine what this is going to look like when I replace the copper with red stain!
Clockwise: G3806C,D,E,F on M370

F is more fluid, thus highlights contours better.
C has 2% copper, the others 3%.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama c6 - Lower COE #2" id="157465" key="R1pRJCSD" date="2022-08-30" codenum="G3806E" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="32.000" tolerance=""/> <recipeline material="Spodumene" amount="11.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="3.000" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="6.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="22.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="5.000" tolerance=""/> <recipeline material="Kaolin" amount="11.000" tolerance=""/> <recipeline material="Bentonite" amount="2.000" tolerance=""/> <recipeline material="Dolomite" amount="8.000" tolerance=""/> <recipeline material="Copper Oxide" amount="4.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2022-08-30 07:38:11
Panama c6 - Lower COE #3
|
Code # G3806F |
| P | Materials | Amt |
|---|---|---|
| Silica | 23.500 | |
| Wollastonite | 7.000 | |
| Kaolin | 10.000 | |
| Strontium Carbonate | 5.000 | |
| Ferro Frit 3110 | 22.000 | |
| Ferro Frit 3249 | 21.000 | |
| Zinc Oxide | 3.000 | |
| Spodumene | 6.000 | |
| Bentonite | 2.500 |
| P | Additions | |
|---|---|---|
| * | Copper Carbonate | 4.000 |
| * | Tin Oxide | 2.500 |
Total:106.50
Auto Unity Formula
|
Si:Al: 10.7:1 6.3 (Molar:6.1) 3.0 |
Notes
This version tests a new concept: Introduce more B2O3 (tripling the amount), boron is a glass super-melter. The downside is more bubbling and more frit is needed, that increases the price. But is also means that less Li2O might be needed.
If the melt fluidity increases enough it will permits increasing Al2O3 and SiO2. That is exactly what has happened. This is running much more, even though the Li2O (a very strong flux) is only half what it was.
The calculated COE is the same as the last one, E. And fails the 300F/icewater test on P300 also. Next step: Increase SiO2 and Al2O3.
Pictures
G3806E and F Flow Test

F is flowing better than any previous, likely because of the higher boron. This will permit adding silica, which will lower thermal expansion even more.
The E version is distinctly lacking in bubbles, this is the best looking flow of anything tested.
Clockwise: G3806C,D,E,F on M370

F is more fluid, thus highlights contours better.
C has 2% copper, the others 3%.
G3806E (left), G3806F (right)

E uses 4% copper oxide, F 4% copper carbonante.
On Polar Ice casting body, cone 6.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama c6 - Lower COE #3" id="157668" key="KvgzKTXc" date="2022-07-21" codenum="G3806F" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="23.500" tolerance=""/> <recipeline material="Wollastonite" amount="7.000" tolerance=""/> <recipeline material="Kaolin" amount="10.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="5.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="22.000" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="21.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="3.000" tolerance=""/> <recipeline material="Spodumene" amount="6.000" tolerance=""/> <recipeline material="Bentonite" amount="2.500"/> <recipeline material="Copper Carbonate" amount="4.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2022-07-21 18:36:56
Panama c6 - Lower COE #7
|
Code # G3806K |
| P | Materials | Amt |
|---|---|---|
| Silica | 21.500 | |
| Bentonite | 2.000 | |
| Kaolin | 7.000 | |
| Ferro Frit 3249 | 17.000 | |
| Zinc Oxide | 2.500 | |
| Fusion Frit F-493 | 7.500 | |
| Fusion Frit F-524 | 42.500 |
| P | Additions | |
|---|---|---|
| * | Copper Carbonate | 4.000 |
| * | Tin Oxide | 2.500 |
Total:106.50
Auto Unity Formula
|
Si:Al: 10.4:1 5.8 (Molar:5.5) 1.1 Cost 1.58 per kg |
Notes
This version makes a bold change: Doubling the B2O3. And raises the SiO2 somewhat.
And another bold change: Sourcing Li2O and SrO from Fusion frits. This doubles the percentage of frit in the recipe. Why? I want a consistently crystal-clear glass. The very best way to get that is to reduce materials that have a weight loss on firing (like strontium, kaolin) and maximize frit content.
This is certainly the most fluid so far.
It is an exciting step because the Al2O3 and SiO2 are high (making it likely to be durable) but the fluidity leaves room to raise them much higher! That will nudge the thermal expansion even lower.
The 7% koalin seems to be adequate for suspending the slurry because it is assisted by 2% bentonite.
Pictures
G3806K on M370, Coffee clay

Crystal clear, running nicely.
G3806K ball pool - some bubbles

Now that is melt fluidity! G3806K with and without copper

This is clearly running much better than any previous. Much fewer bubbles. Even some crystallization on lower flow. More Al2O3 will reduce that.
G3806K on Polar Ice - really running

It is pooled in the bottom of the mug. Polar Ice.
This survived 325F to ice water without crazing!
G3806K on coffee clay

XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama c6 - Lower COE #7" id="158673" key="Bdabd9go" date="2019-08-08" codenum="G3806K" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="21.500" tolerance=""/> <recipeline material="Bentonite" amount="2.000" tolerance=""/> <recipeline material="Kaolin" amount="7.000" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="17.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.500" tolerance=""/> <recipeline material="Fusion Frit F-493" amount="7.500" tolerance=""/> <recipeline material="Fusion Frit F-524" amount="42.500" tolerance=""/> <recipeline material="Copper Carbonate" amount="4.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2019-08-08 14:36:32
C6 Fluid Clear Final Recipe #10
|
Code # G3806N |
| P | Materials | Amt | |
|---|---|---|---|
| Silica | 17.500 | 17.33% | |
| Bentonite | 1.000 | 0.99% | |
| Kaolin | 17.000 | 16.83% | |
| Ferro Frit 3249 | 16.000 | 15.84% | |
| Zinc Oxide | 2.500 | 2.48% | |
| Fusion Frit F-524 | 40.000 | 39.60% | |
| Fusion Frit F-493 | 7.000 | 6.93% |
| P | Additions | ||
|---|---|---|---|
| * | Black Copper Oxide | 4.000 | 3.96% |
| * | Tin Oxide | 2.500 | 2.48% |
Total:107.50
RO Unity Formula
|
Si:Al: 7.5:1 5.8 (Molar:5.5) 2.2 Cost 1.48 per kg |
Notes
In an intervening step I increased the Al2O3 from .38 to .45. It this one to 0.54 (almost double the amount of Al2O3 from G3806C). Yet it maintains fluidity. And SiO2 is 25% higher! And the thermal expansion calculates to an incredible 5.8 (compared to the original Panama blue which was 7.7)!
This recipe delivers the key fluxing oxides SrO, KNaO, MgO and Li2O from frits, giving an LOI of only 2.2. This could be the reason for such good melt fluidity with such high Al2O3.
Orange-peeling of the surface was an issue on some pieces, maybe from the high Al2O3. But it is undeniably super fluid.
You may not be able to get these frits easily (although not shown here, I want to use Fusion F-69 instead of Ferro 3249, being intended for ceramics it is more reliable).
If you cannot get the frits right now, what is your best option until you can? Consider making the G3806E or G3806F. Make sure they fit your clay body. Do a 300F to ice-water test to be sure.
Pictures
Lemon slice test on G3806N

Left this for 24 hours. Wrapped up in stretch wrap.
No indication of leaching.
G3806N P300 mug

This withstands a 325F to ice water test without crazing on P300, a very difficult-to-fit-glazes-to body.
G3806N on M370, M340, M390, Coffee

Cone 6 drop and soak, slow cool firing.
Jackpot! Looks great. Appears to be running less yet still exhibits the dramatic color changes associated with its mobility on contoured surfaces.
G3806N flow test

G2934Y, G2926B, G3806N on L4197

Slow cool. Cone 6. Sitting for three weeks.
No apparent crazing.
G2934Y, G2926B, G3806N on L4198

Cone 6. Slow cool. Sitting for 3 weeks.
G2926B is crazing.
G2926B, G2934Y on L4199

Cone 6 slow cool. Sitting for 3 weeks.
G2926B has crazed.
G2934Y, G2926B, G3806N on L4200

Cone 6 slow cool. Sitting for 3 weeks.
G2926B has crazed.
G2934Y, G2926B, G3806N on L4201

Cone 6 slow cool. Sitting for about 3 weeks.
G2926B is crazing.
G2934Y, G2926B, G3806N on L4202

Cone 6, slow cool. Sitting for 3 weeks.
No apparent crazing.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="C6 Fluid Clear Final Recipe #10" id="159060" key="Xqo32XXa" date="2022-07-08" codenum="G3806N" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="17.500" tolerance=""/> <recipeline material="Bentonite" amount="1.000" tolerance=""/> <recipeline material="Kaolin" amount="17.000" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="16.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.500" tolerance=""/> <recipeline material="Fusion Frit F-524" amount="40.000" tolerance=""/> <recipeline material="Fusion Frit F-493" amount="7.000" tolerance=""/> <recipeline material="Black Copper Oxide" amount="4.000" added="true"/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2022-07-08 18:16:12
G3806N1 + 2% Zircopax
|
Code # L4273 |
| Materials | Amt | Units |
|---|---|---|
| Silica | 18.500 | |
| Kaolin | 15.500 | |
| Fusion Frit F-69 | 17.000 | |
| Zinc Oxide | 2.000 | |
| Fusion Frit F-524 | 36.000 | |
| Fusion Frit F-493 | 6.500 | |
| Wollastonite | 1.500 | |
| Ferro Frit 3110 | 3.000 |
| Additions | Units | |
|---|---|---|
| Zircopax | 2.000 | GM |
*Missing units are assumed to be R
Total:102.00 (R)
Auto Unity Formula
|
Si:Al: 8.4:1 5.8 (Molar:5.5) 1.9 Cost 1.34 per kg |
Notes
Switched to Fusion Frit F-69 for MgO.
Added some Frit 3110 to supply more Na2O.
We have mixed many colors of this and done lots of testing. As noted in N, it appears the Al2O3 is too high. The next version will reduce it and increase the SiO2. This will produce an Si:Al ratio more in keeping with an ultra gloss.
Minute bubbles can be observed in double dipped area at top of tile, but are very hard to see with the 8x lab microscope. On the front of the tile where glaze is allowed to pool in incised areas of tile, there is also the occasional minute bubble. Smoother looking surface on back of tile when comparing to G3806N1 glazed tile with light reflection.
Pictures
G3806N1 flow tests - Tin vs. Zircon

Flow test and test tile (P300 P6805) fired C6DHSC schedule. Flow test balls 9 grams.
This appears to be an improvement over the previously tested G3806N1 base glaze.
Tile was dropped in ice water at 310F. No crazing on first day.
Test tile glaze thickness may be slightly thinner than original G3806N1 test tile fired last week.
C6DHSC firing schedule

stain additions at 10%, zircopax at 2% PLC6DS

Tiles are P300
Important reference notes for the Mason Stains:
6021 Dark Red: May be used with or without zinc.
6304 Violet: Do not use Zinc in glaze. (This did not really effect color developement like the zinc has done to some of the other Mason stains).
6600 Black: May be used with or without Zinc.
6666 Cobalt Free Black: Do not use Zinc in glaze. As you can see here, the zinc has turned the test tile a chocolate brown color.
Do not have any reference from Cerdec in regards to use of their stains, but color development is similar to when used with other base glazes.
Fired May12/20 and observed once again March 4/2021 and none of these tiles have crazed.
stain additions at 10%, zircopax at 2% PLC6DS

Tiles are P300
This is the flip side of the tiles with the notes.
Fired May12/20 and observed once again March 4/2021 and none of these tiles have crazed.
3% tin oxide addition, no zircopax

Tile is P300
Fired PLC6DS schedule May 12/2020 and observed March 4/2021 and glaze has not crazed.
Tile is Polar Ice Stains were brus

Tile is Polar Ice
Stains were brushed on, not very good or consistent application between the three.
Important Mason Reference notes:
6001 Alpine Rose: Do not use Zinc in glaze. Glaze must contain 6.7-8.4% Calcium Oxide?, or 12-15% CaCO3?
6003 Crimson: Same notes as above Alpine Rose.
6020 Managanese Alumina Pink: This is a body stain, did not really expect this to work.
It appears the Zinc Oxide in the base glaze has ruined the color development of the 6001 and 6003 stains as Mason said it would?
C6DHSC (slow cool)

Joe: Stain and glaze combinations are the same as previous test photos, included with tile of 3% tin oxide and tile of base glaze with no zircopax addition. Tiles are P300 clay.
Tile double dipped at top like other firings, and glaze application was quite thin in my opinion.
Firing schedule has not changed final outcome in my opinion.
These tiles were fired June 4/2020 and observed once again on March 4/ 2021 and all mason stained tiles are o.k., but the copper oxide, tin oxide colored tile is crazing. The two white tiles, one is the base clear, and the other with a tin oxide addition and neither crazed as well.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="G3806N1 + 2% Zircopax" id="176749" key="ffvQC3HT" date="2020-06-22" codenum="L4273" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Silica" amount="18.500" tolerance=""/> <recipeline material="Kaolin" amount="15.500" tolerance=""/> <recipeline material="Fusion Frit F-69" amount="17.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.000" tolerance=""/> <recipeline material="Fusion Frit F-524" amount="36.000" tolerance=""/> <recipeline material="Fusion Frit F-493" amount="6.500" tolerance=""/> <recipeline material="Wollastonite" amount="1.500" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="3.000" tolerance=""/> <recipeline material="Zircopax" amount="2.000" unitabbr="GM" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2020-06-22 15:36:12
