Project Name
Compare Cone 6 clear fluid glazes
Project Codenumber
UnAssigned
Notes
This project compares a variety of fluid-melt (runny) cone 6 base base glazes. Why do this? The initial objective was to create a good copper blue. But that was extended to create a fluid-melt base to which other oxide and stains can be added to make brilliant colors. Objectives:
-Learn the tradeoffs between these and a more stable melt bases (like G2926B).
-Find one that does not craze on common bodies.
-Compare their tendency to trap bubbles (in various types of bodies), the smoothness of their surfaces.
-Compare their reactions to various colorants, opacifiers.
-Compare their melt flows.
-Compare their behavior as slurries
These are the initial glazes. Some lived as colored glazes, but I removed the colorant to just look at the base clear. I had to adjust two of them immediately, they would not suspend in the bucket.
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
I chose an initial winner: G3806B (it is a second-step adjustment to the original to increase the clay content, while holding chemistry the same, to give it better working properties). I may revisit other bases (I like the Campana and 3808A also).
This project underscores the fact that textbook recipes (which the initial ones all were) need to be tested and adjusted to bring into our circumstances. Invariably they have one or more of the following problems: they settle, dry-crack, dust, gel, craze, shiver, blister, pinhole, leach or do not fire to a smooth surface. But in comparing the chemistry, materials and firing of a number of different ones one can pick the best and adjust that to get the final mix.
Low Zinc High Feldspar Fritless base
Roberta Capogna Bateman - cone 6
|
Code # G3814 |
| Materials | Amt |
|---|---|
| Custer Feldspar | 53.000 |
| Silica | 17.000 |
| Whiting | 15.000 |
| EP Kaolin | 6.000 |
| Zinc Oxide | 5.000 |
| Talc | 4.000 |
| Additions | |
|---|---|
| Tin oxide | 1.000 |
| Copper Carbonate | 1.000 |
Total:102.00
Auto Unity Formula
|
Si:Al: 8.8:1 7.4 (Molar:6.9) 8.2 Cost 0.16 per kg |
Notes
This does not have the brilliant gloss the other fluid melt recipes in the comparison have have. The amount of entrained bubbles on the brown stoneware are evidence of this.
But it does melt surprisingly well considering it contains no boron and only 5% zinc. With only a small amount of boron the degree of melt could likely be improved.
This glaze is really lacking in clay so it must be very difficult to suspend in the bucket.
Pictures
G3814 Low Zinc High Feldspar Fritless base

G3814 Low Zinc High Feldspar Fritless base melt balls

Top: G3814
G2926B Plainsman whiteware base
L3808A high B2O3 fluid clear
G3813 Campana clear
At: six on red, buff, white bodies

Although this melts well enough to look good on the buff and white burning clays, the melt is stiff enough that lots of bubbles are trapped in the red burning one
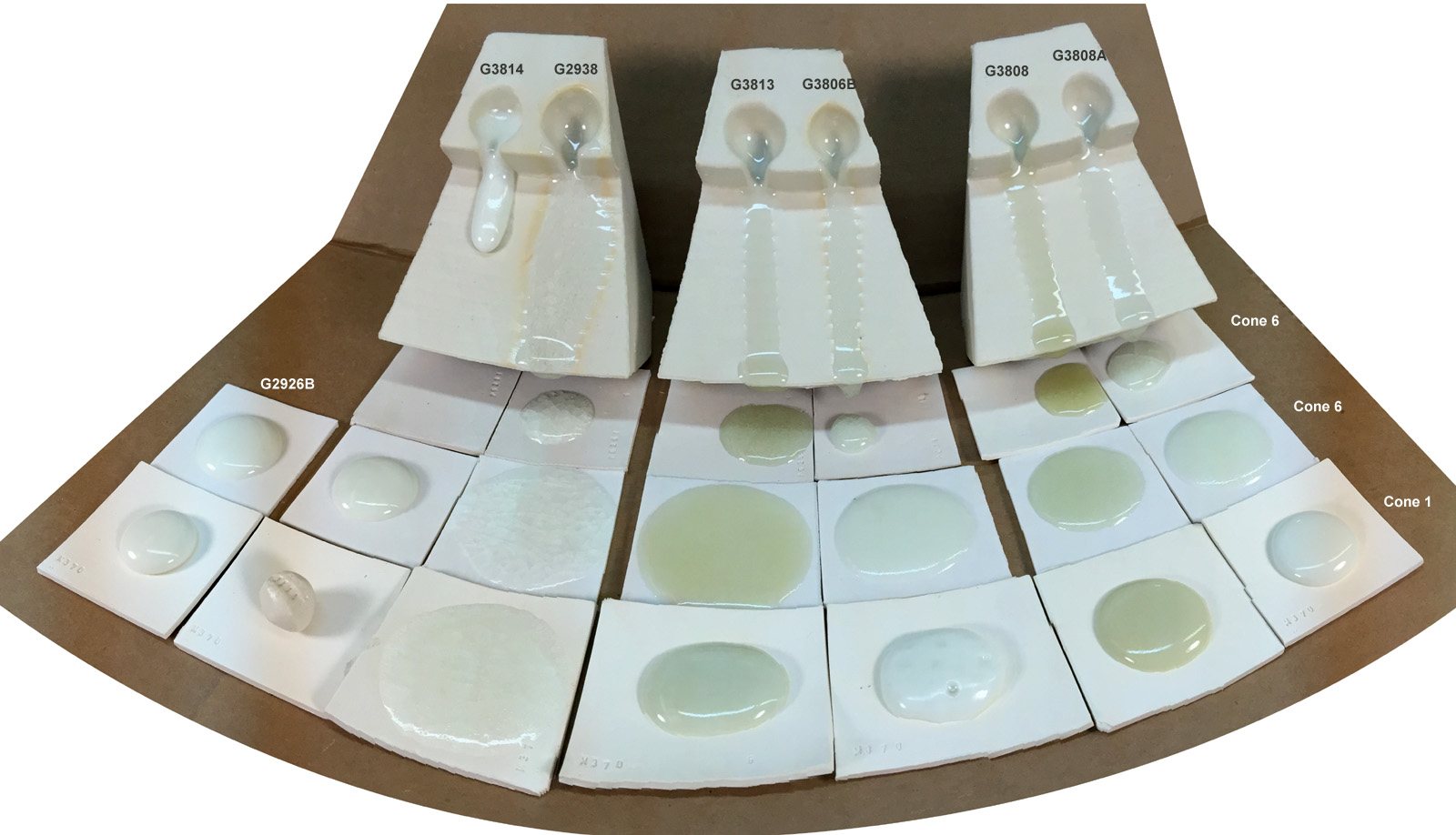
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents
The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Low Zinc High Feldspar Fritless base" keywords="Roberta Capogna Bateman - cone 6" id="74784" key="nWn12np5" date="2015-07-06" codenum="G3814" email="robertabateman@me.com"> <recipelines> <recipeline material="Custer Feldspar" amount="53.000" tolerance=""/> <recipeline material="Silica" amount="17.000" tolerance=""/> <recipeline material="Whiting" amount="15.000" tolerance=""/> <recipeline material="EP Kaolin" amount="6.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="5.000" tolerance=""/> <recipeline material="Talc" amount="4.000" tolerance=""/> <recipeline material="Tin oxide" amount="1.000" added="true"/> <recipeline material="Copper Carbonate" amount="1.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-05, Modified: 2015-07-06 09:05:48
Wright's Water Blue Base
Cone 6
|
Code # G2938 |
| Materials | Amt |
|---|---|
| Lithium Carbonate | 3.000 |
| Strontium Carbonate | 9.000 |
| Ferro Frit 3110 | 59.000 |
| EP Kaolin | 12.000 |
| Silica | 17.000 |
| Additions | |
|---|---|
| Bentonite | 2.000 |
| Copper Carbonate | 5.000 |
Total:107.00
Auto Unity Formula
|
Si:Al: 15.6:1 8.3 (Molar:8.1) 8.2 |
Notes
From David Wright, CM 1998
Has a very high calculated expansion, the degree to which it crazes is thus no surprise. There is even crazing on a 40% silica porcelain.
In the fluid glaze comparison, this base (without the copper) has the smoothest most glassy surface and the lowest bubble population (especially on the buff and brown stonewares).
Pictures
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
CM Top Ten cone 6 glazes - Water Blue

G2938 Wright's Water Blue

From Vivian Pyle
She wants to find a way to eliminate the crazing.
She is using the original recipe with 5% more silica (shown here), but. that is not even close to the amount of change needed to fix this crazing.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Wright&#039;s Water Blue Base" keywords="Cone 6" id="60278" key="PyzbdF1C" date="2024-07-01" codenum="G2938" email="kathryn.kearns@solano.edu"> <recipelines> <recipeline material="Lithium Carbonate" amount="3.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="9.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="59.000" tolerance=""/> <recipeline material="EP Kaolin" amount="12.000" tolerance=""/> <recipeline material="Silica" amount="17.000" tolerance=""/> <recipeline material="Bentonite" amount="2.000" added="true"/> <recipeline material="Copper Carbonate" amount="5.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2014-07-08, Modified: 2024-07-01 13:45:23
Cone 6 Bright Clear - Shaun Mollonga
Gerstley Borate
|
Code # G3808 |
| Materials | Amt |
|---|---|
| Minspar 200 | 40.000 |
| OM4 Ball Clay | 15.000 |
| Gerstley Borate | 35.000 |
| Silica | 10.000 |
Total:100.00
Auto Unity Formula
|
Si:Al: 7.6:1 7.1 (Molar:6.9) 11.8 Cost 0.50 per kg |
Notes
Being used by Shaun Mollonga at Medalta June 2015 on M390. I would like to get the real name of it.
This was compared with three other high fluid melt glossy clear bases.
This recipe takes the high-B2O3 approach to getting a good fluid melt. Low B2O3 expansion appears to be the key to this not crazing even though it has significant KNaO (the good supply of Al2O3 and SiO2 are also likely factors).
That has alot of Gerstley Borate for a cone 6 glaze. With the added ball clay, it is shrinking alot.
This dries really really slowly. It took an hour to cast a flow cone (for others it takes minutes). The GB is desperately hanging on to the water.
It fires to the most amber color of all the recipes. The bubbles are only ones size (no clouds of tiny ones).
Pictures
Bright clear cone 6 with frit

Top row: thick layer of Gerstley, Frit version
Second row: ball flow test Gerstley, Frit version
Bottom: G2926B
Fired to cone 6 on Polar Ice.
Fluid cone 6 clear glazes

These are 10 gram glaze balls are fired down onto tiles to demonstrate melt fluidity and bubbling.
Left: L3808 GB clear from Shaun Mollonga (most fluid).
G3808A fritted recalculation of former (best surface).
G3813 Campana clear (most transparent).
G3806B Panama Blue base.
All of these survived 260F:Icewater test without crazing on M370, M390 and M340.
Campana Clear is the smoothest on M340, Panama is second best.
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
URLs
Cone 6 Fluid Clear Glaze Comparison
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Cone 6 Bright Clear - Shaun Mollonga" keywords="Gerstley Borate" id="74714" key="KcPMk4xN" date="2015-10-08" codenum="G3808"> <recipelines> <recipeline material="Minspar 200" amount="40.000"/> <recipeline material="OM4 Ball Clay" amount="15.000"/> <recipeline material="Gerstley Borate" amount="35.000"/> <recipeline material="Silica" amount="10.000"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2015-10-08 12:09:08
Cone 6 Bright Clear using Frits
|
Code # G3808A |
| Materials | Amt |
|---|---|
| Minspar 200 | 27.984 |
| EP Kaolin | 14.873 |
| Ferro Frit 3134 | 31.746 |
| Silica | 11.111 |
| Ferro Frit 3249 | 11.111 |
| Wollastonite | 3.175 |
Total:100.00
Auto Unity Formula
|
Si:Al: 7.7:1 7.0 (Molar:6.9) 2.3 |
Notes
The recipe started as one having 35% Gerstley Borate, it gelled so badly it was almost unusable and had a very high LOI. Using Insight-live I calculated an recipe having the same chemistry but sourcing the high boron from frits instead. It substitutes kaolin for ball clay also, this should suspend it better.
With an SG of 1.48 it was pretty watery but responded well to Vinegar and especially Epsom Salts (see link).
This has the second glossiest surface. However it is crazing on M370. A little zinc in place of some KNaO would likley increase the flow a little and reduce the expansion.
Pictures
Bright clear cone 6 with frit

Top row: thick layer of Gerstley, Frit version
Second row: ball flow test Gerstley, Frit version
Bottom: G2926B
Fired to cone 6 on Polar Ice.
Various fluidities cone 6 clear

Left: 2926b
Center: G3808
Right: G3808A
G3808A bubbles in glaze

G3814 Low Zinc High Feldspar Fritless base melt balls

Top: G3814
G2926B Plainsman whiteware base
L3808A high B2O3 fluid clear
G3813 Campana clear
Fluid cone 6 clear glazes

These are 10 gram glaze balls are fired down onto tiles to demonstrate melt fluidity and bubbling.
Left: L3808 GB clear from Shaun Mollonga (most fluid).
G3808A fritted recalculation of former (best surface).
G3813 Campana clear (most transparent).
G3806B Panama Blue base.
All of these survived 260F:Icewater test without crazing on M370, M390 and M340.
Campana Clear is the smoothest on M340, Panama is second best.
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
Cone 6 on M370 - 15 minute soak

blisters on the ventilation hole side of a mug.
G3808A highly melt fluid clear on a red body

Has extenal picture also
This is Plainsman M390 fired to cone 6 oxidation. The firing was soaked, dropped 100F and soaked again, then 108F/hr cooled to 1400F. Highly fluid clear glazes work much better on bodies like this (not finely ground, containing high iron clays) because they are able to pass the extra bubbles generated as particles decompose (and create gases) during firing. However there is a danger that blisters will form where the glaze is thicker, and of course, that it will run off the ware onto the shelf. So care needs to be taken to apply it thinly.
Compare four clear bases for copper blue

Has extenal picture also
The flow testers at the back and the melt-down-balls in from of them have 1% copper carbonate. The glazed samples in the front row have 2% copper carbonate. L3806B, an improvement on the Panama Blue recipe, has the best color and the best compromize of flow and bubble clearing ability.
G3808A vs 2926B flow test

URLs
How to gel a glaze..pension properties
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Cone 6 Bright Clear using Frits" id="74715" key="wu8NJk6Q" date="2015-07-30" codenum="G3808A"> <recipelines> <recipeline material="Minspar 200" amount="27.984" tolerance=""/> <recipeline material="EP Kaolin" amount="14.873" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="31.746" tolerance=""/> <recipeline material="Silica" amount="11.111" tolerance=""/> <recipeline material="Ferro Frit 3249" amount="11.111" tolerance=""/> <recipeline material="Wollastonite" amount="3.175" tolerance=""/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2015-07-30 21:55:14
Campana Cone 6 Transparent Glaze
3134, Spodumene, Zinc
|
Code # G3813 |
| Materials | Amt |
|---|---|
| Spodumene | 11.000 |
| Ferro Frit 3134 | 21.000 |
| EP Kaolin | 20.000 |
| Silica | 20.000 |
| Wollastonite | 20.000 |
| Zinc Oxide | 8.000 |
Total:100.00
Auto Unity Formula
|
Si:Al: 8.7:1 6.7 (Molar:6.8) 3.0 |
Notes
This is a popular fluid-melt cone 6 base recipe. We first compared it with 4 others in an effort to produce a fluid-melt, super-gloss, low-expansion transparent base. This appears to be the 20x5 recipe with Custer feldspar switched for a mix of zinc and spodumene (combining the power of lithia, zinc and boron fluxes to achieve a very low calculated thermal expansion, it does not craze on any of the bodies tested). Interestingly, because it has lots of melt fluidity it could likely accept more Al2O3 and SiO2 and yet still fire glossy (meaning the expansion could be taken still lower).
The bubble population is a concern.
Equal weights of water and powder creates about 1.47 SG. To get it to 1.45 SG thus requires more water than powder. It creates alot of bubbles in the slurry itself (from the Spodumene, wash it first to reduce these). The powder resists wetting. It responds quickly to vinegar.
We ended up choosing the Panama base over this one because of the higher bubble population in the melt and the issues that Spodumene creates with the slurry. But if you need a lower thermal expansion (the G3806C crazes on your clay body), then this one is worth trying.
Pictures
Compared with G2926B

These are 10 gram balls melted down onto a tile to demonstrate fluidity, clarity and bubbling.
Left is our standard cone 6 glaze.
Right: G2813.
It is flowing significant more, but not as much more as some others I am testing right now.
Bottom: G3813 on a Polar Ice porcelain tile. It is high glossy, not crazing out of the kiln.
G3813 16x closeup of entrained bubbles

Others also had bubbles, but there are alot here. And they are all the same size (in clouds). Although the photo does not show it clearly, hundreds of these are breaking at the surface, leaving dimples. This was fired by drop-100F-an-soak. This would do better in clearing itself with thinner application.
G3814 Low Zinc High Feldspar Fritless base melt balls

Top: G3814
G2926B Plainsman whiteware base
L3808A high B2O3 fluid clear
G3813 Campana clear
Fluid cone 6 clear glazes

These are 10 gram glaze balls are fired down onto tiles to demonstrate melt fluidity and bubbling.
Left: L3808 GB clear from Shaun Mollonga (most fluid).
G3808A fritted recalculation of former (best surface).
G3813 Campana clear (most transparent).
G3806B Panama Blue base.
All of these survived 260F:Icewater test without crazing on M370, M390 and M340.
Campana Clear is the smoothest on M340, Panama is second best.
Campana clear on Plainsman M370,340,390

These were ice water tested at 260F and did not craze. Coverage is defect free on all three clays.
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
Compare four clear bases for copper blue

Has extenal picture also
The flow testers at the back and the melt-down-balls in from of them have 1% copper carbonate. The glazed samples in the front row have 2% copper carbonate. L3806B, an improvement on the Panama Blue recipe, has the best color and the best compromize of flow and bubble clearing ability.
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Campana Cone 6 Transparent Glaze" keywords="3134, Spodumene, Zinc" id="75226" key="zvNdSmAJ" date="2018-03-19" codenum="G3813" email="strawdogs@hotmail.com"> <recipelines> <recipeline material="Spodumene" amount="11.000" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="21.000" tolerance=""/> <recipeline material="EP Kaolin" amount="20.000" tolerance=""/> <recipeline material="Silica" amount="20.000" tolerance=""/> <recipeline material="Wollastonite" amount="20.000" tolerance=""/> <recipeline material="Zinc Oxide" amount="8.000" tolerance=""/> </recipelines> </recipe> </recipes>
Born: 2015-06-23, Modified: 2018-03-19 15:58:30
Panama Blue 3 - Copper Carbonate
3110, 3134, zinc, Sr
|
Code # G3806B |
| Materials | Amt |
|---|---|
| Custer Feldspar | 11.500 |
| Silica | 20.000 |
| Whiting | 1.000 |
| Kaolin | 15.000 |
| Dolomite | 8.000 |
| Strontium Carbonate | 4.000 |
| Ferro Frit 3110 | 29.500 |
| Ferro Frit 3134 | 7.500 |
| Zinc Oxide | 2.500 |
| Additions | |
|---|---|
| Tin Oxide | 2.500 |
| Copper Carbonate | 2.000 |
Total:103.50
Auto Unity Formula
|
Si:Al: 10.8:1 7.5 (Molar:7.3) 8.1 Cost 0.01 per kg |
Notes
After a year of storage we found a considerable amount of hard dark precipitate lumps stuck to the bottom and walls of glaze bucket. Very hard and had to scrape off with fettling knife. This could be because of solubility of Frit 3110.
Pictures
Fluid cone 6 clear glazes

These are 10 gram glaze balls are fired down onto tiles to demonstrate melt fluidity and bubbling.
Left: L3808 GB clear from Shaun Mollonga (most fluid).
G3808A fritted recalculation of former (best surface).
G3813 Campana clear (most transparent).
G3806B Panama Blue base.
All of these survived 260F:Icewater test without crazing on M370, M390 and M340.
Campana Clear is the smoothest on M340, Panama is second best.
Fluid cone 6 clear glaze comparison

Top are 10 gram balls melted down onto a tile to demonstrate melt fluidity and bubble populations.
Second row: Plainsman M370 whiteware
Third row: Plainsman M340 buff stoneware
Fourth row: Plainsman M390 red stoneware
Left to right:
G3814 - not melting as well
G2938 - Water blue base
G3808 - High Gerstley Borate base
G3808A - 3808 using frits instead
G3813 - Campana base
G3806B - Panama base
Cone 6 High Fluid Melt Transparents

The chemistry of these glazes falls outside typical cone 6 boron, soda, calcia, magnesia chemistry. Why? To achieve higher melt fluidity for a more brilliant surface and for more reactive response with colorant and variegator additions. Classified by most active fluxes they are:
G3814 - Moderate zinc, no boron
G2938 - High-soda+lithia+strontium
G3808 - High boron+soda (Gerstley Borate based)
G3808A - 3808 chemistry sourced from frits
G3813 - Boron+zinc+lithia
G3806B - Soda+zinc+strontium+boron (mixed oxide effect)
Compare four clear bases for copper blue

Has extenal picture also
The flow testers at the back and the melt-down-balls in from of them have 1% copper carbonate. The glazed samples in the front row have 2% copper carbonate. L3806B, an improvement on the Panama Blue recipe, has the best color and the best compromize of flow and bubble clearing ability.
2% Copper carbonate in two different cone 6 copper-blues

The top base glaze has just enough melt fluidity to produce a brilliant transparent (without colorant additions). However it does not have enough fluidity to pass the bubbles and heal over from the decomposition of this added copper carbonate! Why is the lower glaze passing the bubbles? How can it melt better yet have 65% less boron? How can it not be crazing when the COE calculates to 7.7 (vs. 6.4)? First, it has 40% less Al2O3 and SiO2 (which normally stiffen the melt). Second, it has higher flux content that is more diversified (it adds two new ones: SrO, ZnO). That zinc is a key to why it is melting so well and why it starts melting later (enabling unimpeded gas escape until then). It also benefits from the mixed-oxide-effect, the diversity itself improves the melt. And the crazing? The ZnO obviously pushes the COE down disproportionately to its percentage (although there is further to go because it is crazing somewhat).
Copper Blue G8306C using copper carbonate, oxide

Right is G3806C, an adjustment to drop the thermal expansion of B. It does this by trading some of the high-expansion KNaO for a mix of MgO, ZnO and SrO. These is an improvement but it still could craze over time on high-kaolin or low silica porcelains.
One more change: The one on the right uses 2% Copper Oxide instead of 2% Copper Carbonate (left). Both also add 2.5% tin oxide. Strangely the color is only slight darker (the oxide is a more concentrated form of copper than the carbonate).
XML (to paste into Insight)
<?xml version="1.0"?> <recipes version="1.0" encoding="UTF-8"> <recipe name="Panama Blue 3 - Copper Carbonate" keywords="3110, 3134, zinc, Sr" id="75240" key="rB1P8Epz" date="2017-01-07" codenum="G3806B" email="untdkm@sasktel.net"> <recipelines> <recipeline material="Custer Feldspar" amount="11.500" tolerance=""/> <recipeline material="Silica" amount="20.000" tolerance=""/> <recipeline material="Whiting" amount="1.000" tolerance=""/> <recipeline material="Kaolin" amount="15.000" tolerance=""/> <recipeline material="Dolomite" amount="8.000" tolerance=""/> <recipeline material="Strontium Carbonate" amount="4.000" tolerance=""/> <recipeline material="Ferro Frit 3110" amount="29.500" tolerance=""/> <recipeline material="Ferro Frit 3134" amount="7.500" tolerance=""/> <recipeline material="Zinc Oxide" amount="2.500" tolerance=""/> <recipeline material="Tin Oxide" amount="2.500" added="true"/> <recipeline material="Copper Carbonate" amount="2.000" added="true"/> </recipelines> </recipe> </recipes>
Born: 2015-06-02, Modified: 2017-01-07 12:21:33
